Maharashtra Board Class 8 Science Solutions Chapter 7 Metals and Nonmetals
Balbharti Maharashtra State Board Class 8 Science Solutions Chapter 7 Metals and Nonmetals Notes, Textbook Exercise Important Questions and Answers.
Maharashtra State Board Class 8 Science Solutions Chapter 7 Metals and Nonmetals
Class 8 Science Chapter 7 Metals and Nonmetals Textbook Questions and Answers
Exercises
1. Complete the table:
Answer:
| Property of metal | Use in everyday life |
| i. Ductility | i. Gold, silver ornaments |
| ii. Malleability | ii. Aluminium sheets, galvanised sheets |
| iii. Conduction of heat | iii. Stainless steel vessels, copper vessels, boilers |
| iv. Conduction of electricity | iv. Copper wires |
| v. Sonority | v. Brass articles |
2. Identify the odd term.
a. Gold, Silver, Iron, Diamond.
Answer: Diamond. (Others are metals.)
b. Ductility, Brittleness, Sonority, Malleability.
Answer: Brittleness. (Other properties are metallic properties.)
c. Carbon, Bromine, Sulphur, Phosphorus.
Answer: Bromine. (Others are solids.)
d. Brass, Bronze, Iron, Steel.
Answer: Iron. (Others are alloys.)
3. Write scientific reasons.
a. The stainless steel vessels in kitchen have copper coating on the bottom.
Answer: The stainless steel utensils used at home are made of an alloy of iron with carbon, chromium and nickel. Copper is the best conductor of heat as compared to steel. Copper conducts heat uniformly. So the time to cook food in copper vessels is reduced. Therefore, the stainless steel vessels in kitchen have copper coating on the bottom.
b. Copper and brass vessels are cleaned with lemon.
Answer: Metals combine with oxygen to form their oxides. Copper and brass combine with oxygen to form their oxides which forms a blackish layer. Lemon is acidic. When the copper and brass vessels are rubbed with lemon the acid dissolves the oxides formed on the vessels and makes it shine again.
c. Sodium metal is kept in kerosene.
Answer: Sodium is a very reactive metal. If kept in the open, it reacts with atmospheric oxygen and moisture and immediately catches fire. On the other hand, Sodium does not react with kerosene. Therefore in order to avoid it catching fire and thereby avoiding accidents, sodium is kept in kerosene.
4. Answer the following.
a. What is done to prevent corrosion of metals?
Answer: To prevent corrosion of metals, layers of oil, grease, varnish and paint are applied on them. Also plating with another non corroding metal is done. Iron is arrested by zinc plating. Due to these processes the contact of metal surface with air is lost and corrosion cannot occur as the chemical reaction cannot occur.
b. What are the metals that make the alloys brass and bronze?
Answer: The alloy brass is formed from copper and zinc and the alloy bronze is formed from copper and tin.
c. What are the adverse effects of corrosion?
Answer: Gases in the air react with metals in presence of moisture to form metal compounds. This is corrosion. Due to corrosion, metals get damaged and spoilt.
d. What are uses of Noble metals?
Answer:
Uses of Noble metals:
1. Gold, silver and platinum are used to prepare ornaments.
2. Silver used in medicines. (It has antibacterial property).
3. Gold and silver also use to make medals.
4. Gold and silver also used to make few electronic devices.
5. Platinum, palladium metals are used as catalyst.
5. Three experiments to study the process of rusting are given below. Observe the three test tubes and answer the following questions.
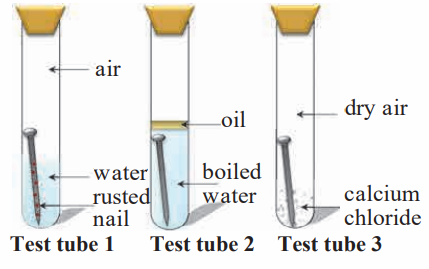
a. Why the nail in the test tube 2 is not rusted?
Answer: Boiled water is free from all gases. The layer of oil in test tube 2 prevents the nail from reacting with the gases in the air. Therefore the nail in test tube 2 is not rusted.
b. Why is the nail in the test tube 1 is rusted highly?
Answer: When the metals come in contact with the gases they react and form metal compounds. In test tube 1 we see that the nail is contact with water as well as air. Gases in the air react with the metal of the nail in presence of moisture to form metal compounds. Therefore the nail in test tube 1 is highly rusted.
c. Would the nail in the test tube 3 get rusted?
Answer: When the metals come in contact with the gases they react and form metal compounds. The calcium chloride in test tube 3 absorbs moisture and makes the air dry. Therefore the nail in test tube 3 does not react with any gases and will not rust.
Project :
How is the ‘Varkha’ or sliver foil used in sweets made ?
Collect the information about which metals are used to make ‘Varkha’.
 JK Academy
JK Academy